Responsible UV protection
Types of UV filters and how they protect us: popular misunderstandings and scientific facts
UV filters are the most important ingredients of sunscreens. They protect us from UV radiation. It is therefore no surprise that there is a high interest in what these UV filters are and how they work. A lot of misinformation circulates on this topic
which can create confusion and uncertainty among consumers rather than provide reliable and helpful information.
Let us have a look at some of these popular misunderstandings and compare them with scientific facts.
Misconception 1: “There are physical (mineral) and chemical UV filters.”
One of the most popular statements on UV filters is the differentiation between “physical” and “chemical” filters, whereas “physical” refers to the two mineral-based filters titanium dioxide and zinc oxide.
This is may lead to wrong conclusions since titanium dioxide and zinc oxide are, like all UV filters, chemical substances. This becomes obvious from the official definition of the international chemicals’ nomenclature and terminology organization IUPAC* , which states that a chemical substance is a form of matter having constant chemical composition and characteristic properties1.
Nevertheless, a correct differentiation can be made between organic chemical UV filters, which are based on carbon, and inorganic chemical UV filters which are not carbon-based (also called mineral filters).
Misconception 2: “Mineral UV filters are natural.”
A typical association connected to the phrase “mineral UV filters” is that these are “natural” compounds. Again, this is a wrong perception. Titanium dioxide and zinc oxide as such occur naturally in a limited amount, but not in a form that can be utilized in sunscreens.
Titanium dioxide and zinc oxide for utilization in sunscreens are chemical modifications of minerals and ores, which are neither allowed for the use in cosmetics nor could they protect against UV radiation. The production processes are energy intensive and require high temperatures and highly reactive chemicals like sulfuric acid and chlorine2, 3. The use of titanium dioxide and zinc oxide in sunscreens requires an additional coating to suppress its photocatalytic activity, i.e., the generation of free radicals which can damage the skin.
Whether a UV filter is mineral or not is not relevant for their main function, namely the protection against UV radiation. This leads us to the next popular misunderstanding.
Misconception 3: “Mineral UV filters act like little mirrors, while chemical UV filters absorb UV light.”
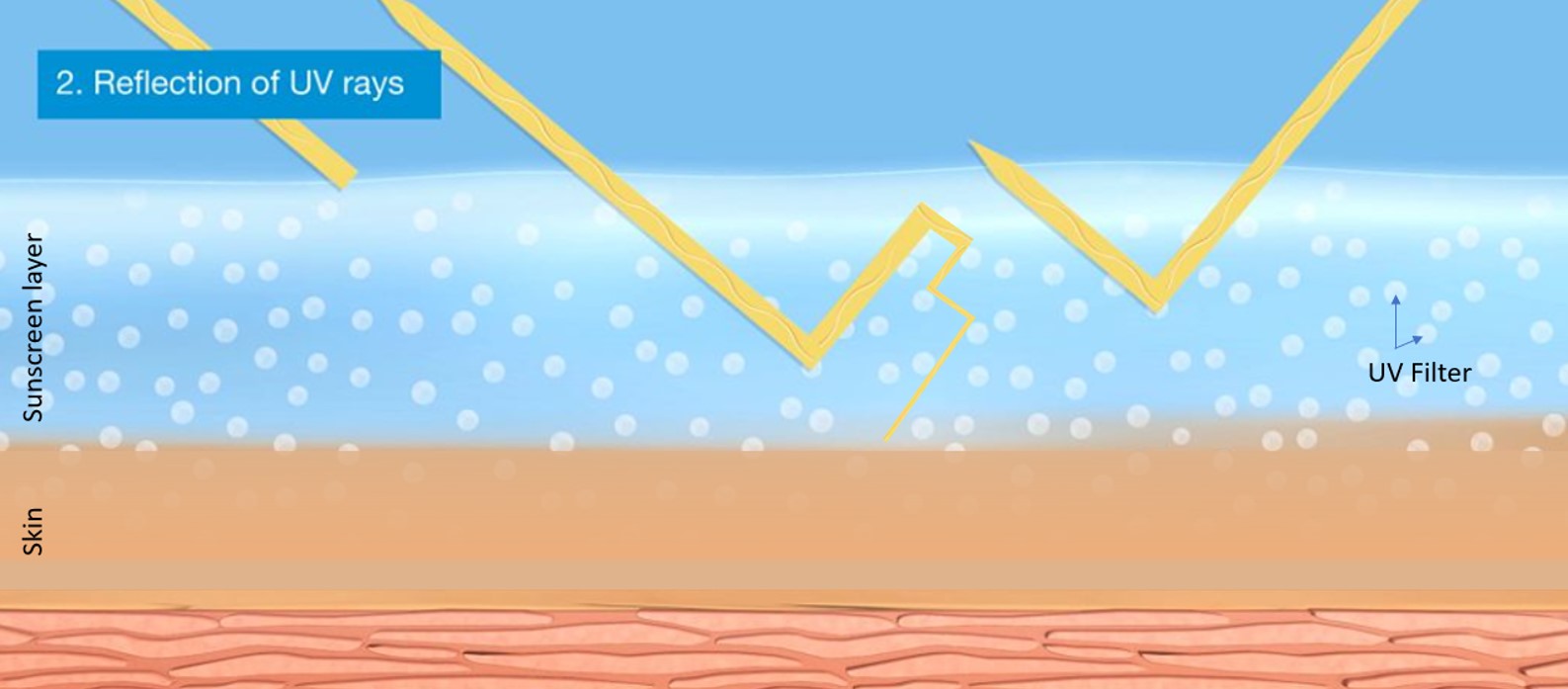
Some UV filters indeed can reflect (“mirror effect”) UV radiation, but this is not an exclusive property of mineral filters alone. All insoluble UV filters – inorganic and organic – are particles (crystals consisting of many molecules) and show this effect.
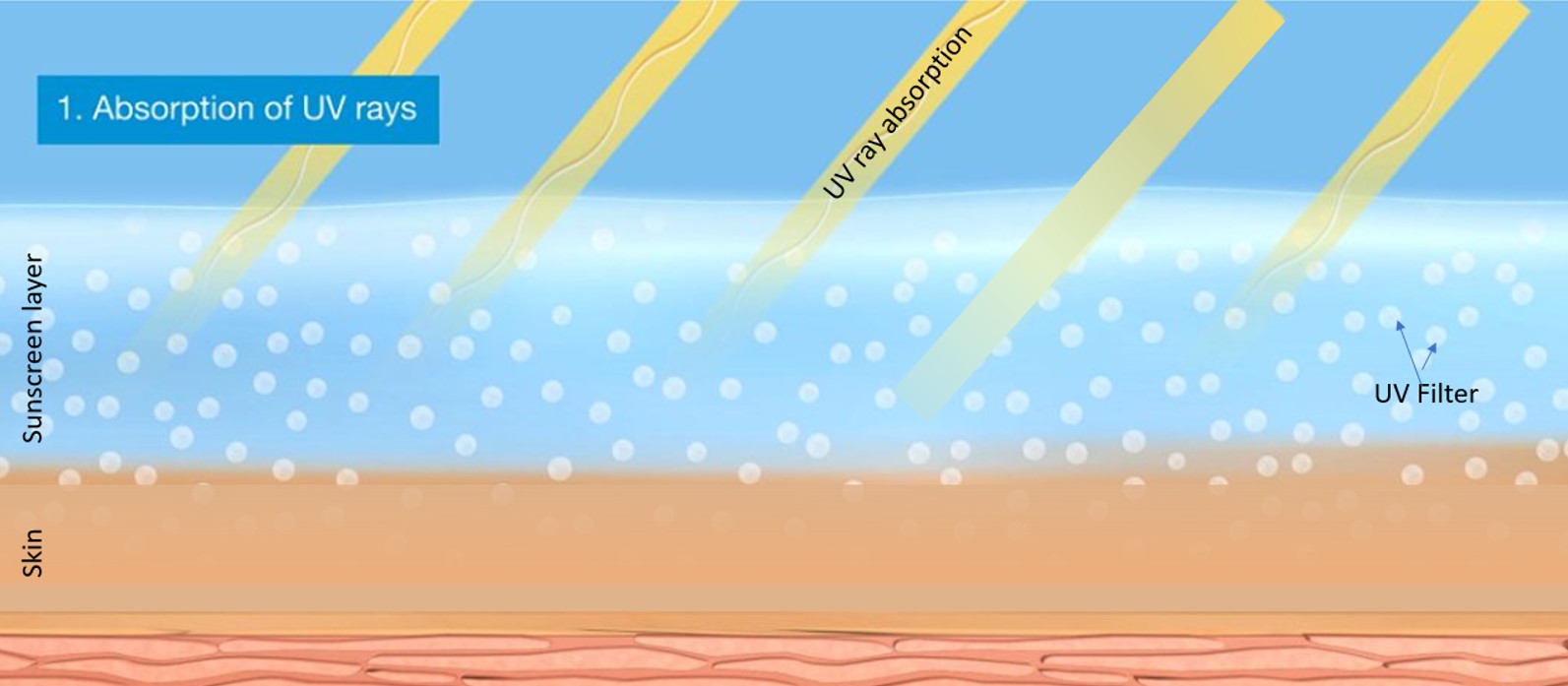
More importantly, the fact that reflection contributes only 10% to the overall protection by a UV filter gives it minor relevance4. The main protection mechanism for all UV filters – organic or inorganic, soluble or insoluble – is called absorption. In this process the UV filters take in the highly energetic UV radiation and release it as warmth.
Figure 1: Protection mechanisms of UV filters
However, inorganic filters are less efficient than most organic filters and therefore typically have to be used in significantly higher concentrations.
Misconception 4: “Natural UV filter means eco-friendly and safe.”
In everyday language, the word “natural” is often used to describe goods that are wholesome and not artificial. There is a misguided notion that natural equals better, safer, healthier and non-toxic: people assume that a “natural” ingredient would possess greater inherent safety for humans and the environment than a chemically synthesized ingredient.
But there is no direct link between naturalness, toxicity, and environmental friendliness. There are lots of natural ingredients that are toxic for humans (e.g., parts of poisonous plants, allergens, etc.) or have a negative environmental impact such as fossil oil by being not biodegradable and toxic to aquatic life.
Conversely, just because an ingredient is synthetic does not make it necessarily a pollutant or toxic for humans. There are “good and bad” natural and synthetic ingredients.
Additionally, other substances stemming from natural sources such as plant or algae extracts, which show similar properties to UV filters by absorbing UV radiation, are already utilized in sunscreen formulations to boost the Sun Protection Factor value.
According to the definition of the Cosmetic Products Regulation [1223/2009], they should be considered as UV filters and should undergo the mandatory risk assessment by the SCCS** for use in sunscreens, based on their toxicological effects.
Finally, it is important to note that the chemical nature allows no conclusion on whether the use of a UV filter is safe for humans or the environment. Therefore, any registered UV filter, organic or inorganic, had to undergo the same strict safety assessment as defined in the Cosmetic Products Regulation [1223/2009].
Explanation
* International Union of Pure and Applied Chemistry
** Scientific Committee on Consumer Safety
Sources
1 Chemical substance - Wikipedia and IUPAC, Compendium of Chemical Terminology, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Chemical Substance". doi:10.1351/goldbook.C01039
2 S. Middlemas et al. / Journal of Cleaner Production 89 (2015) 137-147
3 Zinc oxide - Wikipedia, Porter F (1991). Zinc Handbook: Properties, Processing, and Use in Design. CRC Press. ISBN 978-0-8247-8340-2.
4 Cole C, Shyr T, Ou-Yang H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol Photoimmunol Photomed. 2016;32(1):5-10. doi:10.1111/phpp.12214