Responsible UV protection
Safety Assessment of Cosmetic Ingredients and Products
Prior to placing a cosmetic ingredient or product on the European market, a safety assessment has to be performed to ensure its safe use. It considers all available data for each single ingredient, from physico-chemical properties to toxicity information, and a detailed safety profile is established. This rigorous assessment considering different hazard classes is the “gold standard” worldwide for protecting citizens from potentially negative effects of cosmetic products.
Abstract: Prior to placing a cosmetic ingredient or product on the European market, a safety assessment has to be performed to ensure its safe use. It considers all available data for each single ingredient, from physico-chemical properties to toxicity information, and a detailed safety profile is established.
Information: According to the Cosmetic Products Regulation (CPR), for each cosmetic product on the European market a safety assessment needs to be performed. This assessment has to be carried out by specifically trained toxicologists, who consider each single ingredient of the product in the respective concentration. For three categories of cosmetic ingredients, one of which are UV filters, stricter requirements in the form of a pre-market approval need to be fulfilled. The respective data is evaluated by the Scientific Committee on Consumer Safety (SCCS) on behalf of the European Commission, leading in the end to an inclusion in an annex of the CPR.
The assessment requirements are also specified by the SCCS and consist of 4 steps:
1.) For each cosmetic ingredient, several endpoint specific hazards are determined:
- Acute toxicity
- Skin irritation
- Eye irritation
- Skin sensitization
- Phototoxicity
- (Photo-)Genotoxicity / Carcinogenicity
- Systemic toxicity
- Reproductive toxicity
- ADME1 / Dermal penetration
2.) Identification of a valid No-Observed-Adverse-Effect Level (NOAEL):
The NOAEL represents the highest dose or exposure concentration of a substance for which no significantly harmful outline can be observed.
3.) Determination of a Systemic Exposure Dose (SED):
The SED is derived from the concentration of the substance in the final preparation (meaning the cosmetic product, i.e., sunscreen), the amount of the cosmetic product used during the typical application (i.e., face, whole body, hands, …),
duration of exposure (leave-on, rinse-off, daily, …) and dermal penetration.
4.) Determination of a Margin of Safety (MoS):
By using all this background information, the safety of a certain ingredient in a cosmetic preparation as well as the safety of the complete preparation is calculated. Safety factors are applied to account for the differences between test systems
and humans (i.e., a factor of 10 to account for the variability) and between human individuals (again adding a factor of 10), so that a safety factor of at least 100 must be achieved.
As shown above, all cosmetic ingredients and products entering the market are subjected to a rigorous assessment for different hazard classes in order to protect EU citizens from potentially harmful effects. To increase the margin of safety even further, an additional safety factor of at least 100 must be achieved. With this the EU has already a robust safety assessment, that is the “gold standard” worldwide for protecting its citizens from potentially negative effects of cosmetic products.
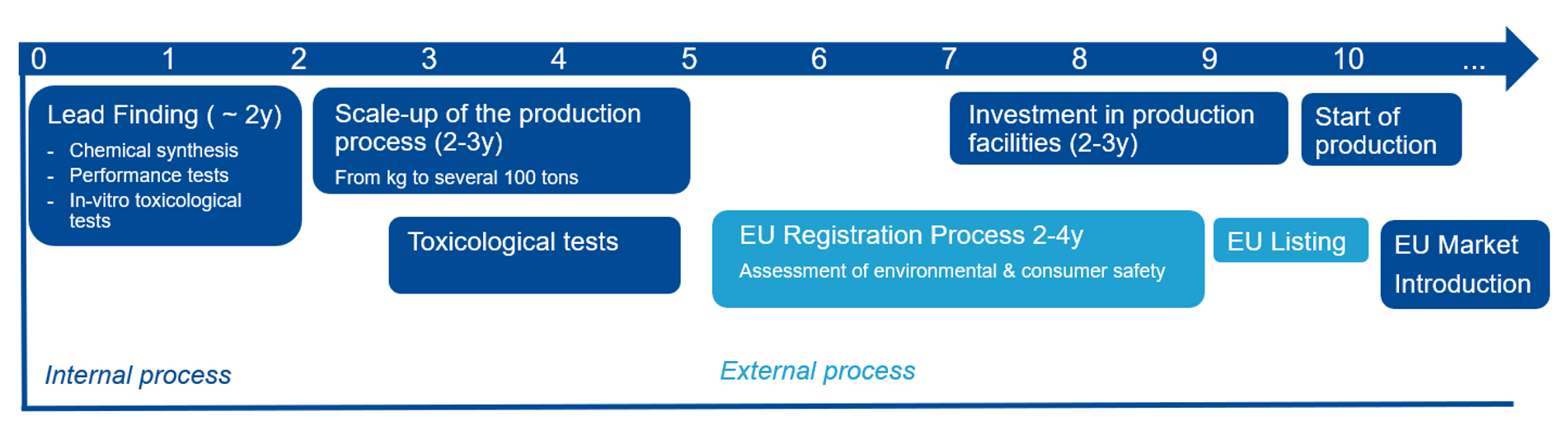
Development timeline for a cosmetic UV filter
Developing a new, safe and high-performance UV filter is time and resource intensive – at least 10 years are required to go through all development steps from basic research, product development and the approval process to an industrial production. A further hurdle posed to the development of new UV filters are conflicting regulatory requirements laid out in the REACH Regulation and the Cosmetic Products Regulation (CPR) regarding animal testing.
Explanation
1 Absorption, Distribution, Metabolism and Excretion